
An example of such a small molecule is hydrogen (H 2). They need less energy to float around freely. Small, spherical molecules that don’t attract one another become a gas more easily than large bulky molecules. These temperatures are different because of how molecules interact, how well they hold onto one another and how well they attract (or repulse) one another. Water ice melts at 0☌ (32☏), but that butter only melts when it’s in a hot pan. Phase transition temperatureĪt which temperature these phase transitions occur is different for every molecule or molecule mixture. Cooling down a gas can transform it into a liquid and cooling it even further makes it into a solid. It’s what happens when bringing water to the boil and continuing to heat it, over time all water will be evaporated. A liquid again can transform into a gas by heating it up. A solid can transform into a liquid by heating up that solid. States of matter can easily transition into one another. Cooking maple syrup, you’re converting liquid into gas! Changing phase In order for molecules to be a gas they need even more energy to travel so freely. These borders are formed by liquids or solids, confining the gases. Instead, gas molecules can travel as far as they want until they hit a border. The molecules aren’t bound anymore by their fellow molecules. In gases molecules get even more freedom. When you pour the olive oil it will pour out of the bottle, but all of it will flow down, pulled down by gravity and end up in a puddle. However, they cannot completely ‘fly’ away from the liquid, they will stay within.Īnother example of a liquid is olive oil at room temperature. In fact, they have enough energy to leave their spot and travel around within the liquid. In a liquid the molecules have a lot more energy. Next up is a liquid, like the water in a glass. If you make a mound of sugar, it will stay in place.

These molecules don’t have enough energy to move away from their spot. They might vibrate back and forth, shiver, etc., but they stay put. In a solid, molecules move, however, they move on their location. There are three* main states of matter: solid, liquid and gas. How freely they’re able to move depends on the state of matter of those molecules. States of matterĮven though molecules move all the time when above that lowest possible temperature, that does not mean they move freely. After enough bumps, all molecules will move at about the same speed and thus have the same temperature!Ī hot cup of water with a tea bag, there’s quite some movement of molecules within the water that is beautifully visualized thanks to the darker coloured tea. If one molecule has a lot more energy (aka, is hotter) than another one (which is colder) it will transfer some of that additional energy to the low energy molecule.

This is because molecules continuously bump into one another. Molecules transfer kinetic energy to one another.If you place a few very hot and a few very cold molecules in the same room, they will become the same temperature over time (assuming an ideal system). For food this is quite irrelevant though since this absolute zero is at -273☌ (-459☏). There is one situation in which molecules would not move at all: at the lowest temperature possible, the absolute zero.
The lower the temperature the lower the kinetic energy of these molecules.
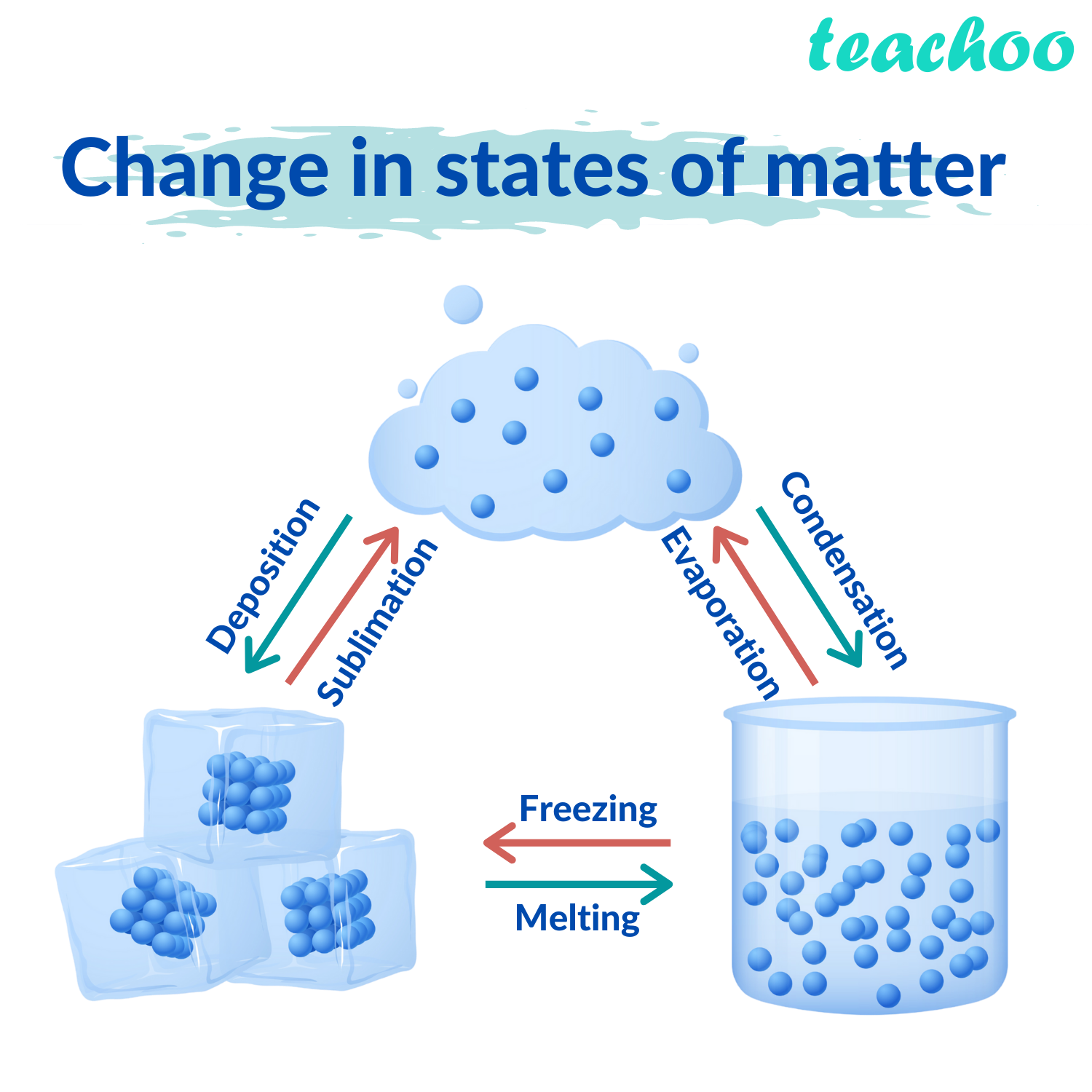
Heat, so a higher temperature, gives the energy to move. The lower the temperature, the less movement. Just how much all the matter around us moves depends on the temperature of that matter. Even though it may not seem like it since it happens at such a small scale, these molecules are moving all the time. Carbohydrates, proteins, fats, they’re all molecules. Molecules form the building blocks of our food as well. The whole world around is is filled with molecules. Without even realizing, you’re continuously switching between three* states of matter: solids, liquids and gases to make delicious food.īut how do those transitions even work? Knowing that will allow you to use them even more to your advantage! Moving molecules – Kinetics You melt butter in a hot pan, boil down a runny stock into a thick syrupy consistency or transform a liquid custard into a solid tub of ice cream. When you prepare food you continuously change the states of our ingredients.


 0 kommentar(er)
0 kommentar(er)
